Our Mission
With A.I., We improve human life
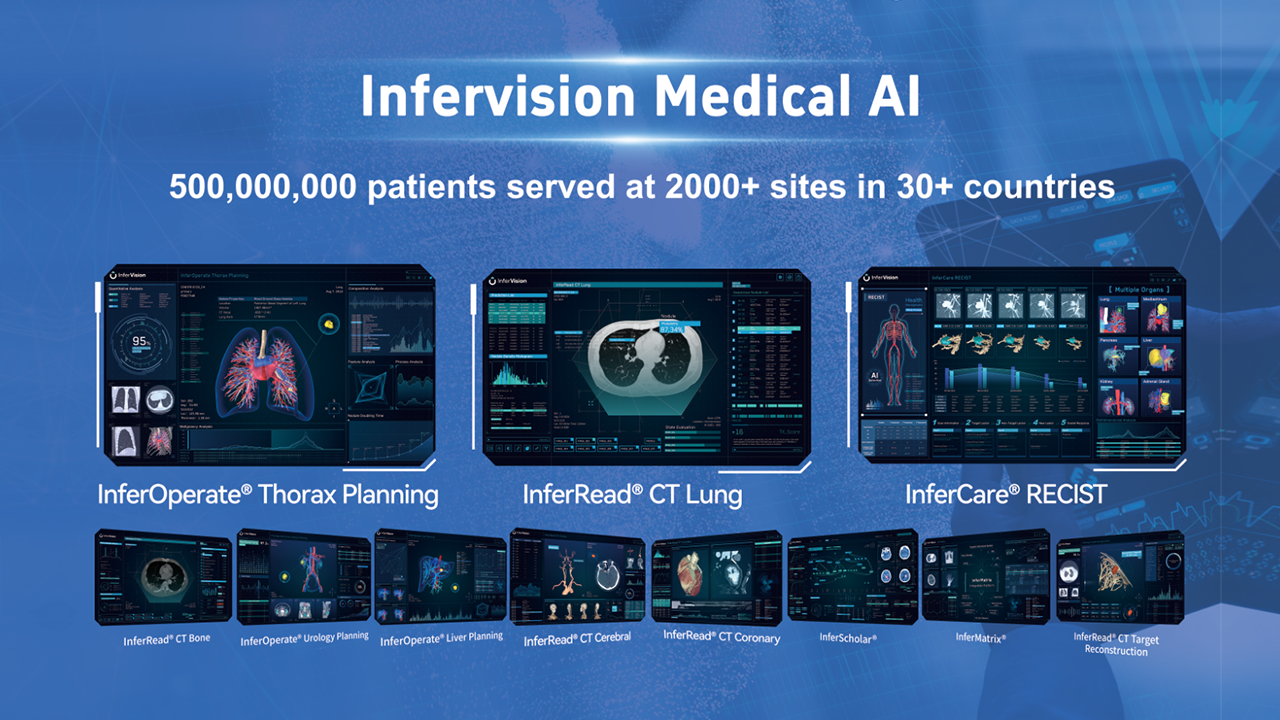
Infervision is a leading AI medical technology company established in 2016. With a focus on improving multi-disciplinary services and operations with AI, Infervision helps healthcare providers in three key areas: disease screening and diagnosis, patient treatment and management, research and development.
Adopting a vertical and horizontal product strategy, Infervision offers a comprehensive series of AI-assisted software to detect a wide range of anomalies such as cancers, infectious diseases, cardiovascular diseases, cerebrovascular diseases and trauma and more. Alongside existing disease screening and diagnosis (InferRead®), intervention and treatment (InferOperate®), patient health management (InferCare®), and teaching and research initiatives (InferScholar®, InferMartrix, InferEducate), Infervision is the trusted partner for hospitals, other medical institutions, governments, doctors and patients across the globe.
Our Journey
2016
Infervision founded
Angel financing completed
Series A and B financing completed
Recognized as National High-Tech Company
2017
2018
US, Europe and Japan offices established
Series B+ and C financing completed
AI going overseas: Participate in world-renowned radiology conferences (ECR, CCR, RSNA, etc.)
2019
2020
Fight against COVID-19 pandemic with AI technology
Four main marketing approvals: CE, PMDA, FDA, NMPA
InferRead® CT Stroke received 510(k) clearance from the FDA
Product procured by the European Commission, and included in the medical devices ordering list under the Global Drug Facility (GDF) hosted by the United Nations Office for Project Services (UNOPS)
2021
2022
InferRead® CT Fracture and InferRead® CT Stroke received the NMPA Class III medical device registration
Product selected into procurement list of NHS
InferRead® CT Target Reconstruction, InferRead® CTA Coronary, and InferOperate® Thorax Planning received NMPA Class III medical device registration
InferRead® DR Chest received MDR CE and UKCA marking
Product procured by the UN to East Europe and Central Asia
2023
2024
Partnered with SimonMed, one of the largest outpatient medical imaging providers in the US
InferOperate® Liver Planning and Urology Planning received NMPA Class lll medical device registration
To be continued…
Latest news
Subscribe to know first
Receive monthly news and insights in your inbox. Don't miss out!

UK
London, N1 7GU, UK
Europe
Wiesbaden, 65189, Germany
US
New Jersey