Infervision has received U.S. Food and Drug Administration 510(k) clearance for its InferOperate Suite, making it the first surgical planning artificial intelligence solution cleared for use in all four of the world’s largest regulatory jurisdictions: the United States, the European Union, the United Kingdom, and China.
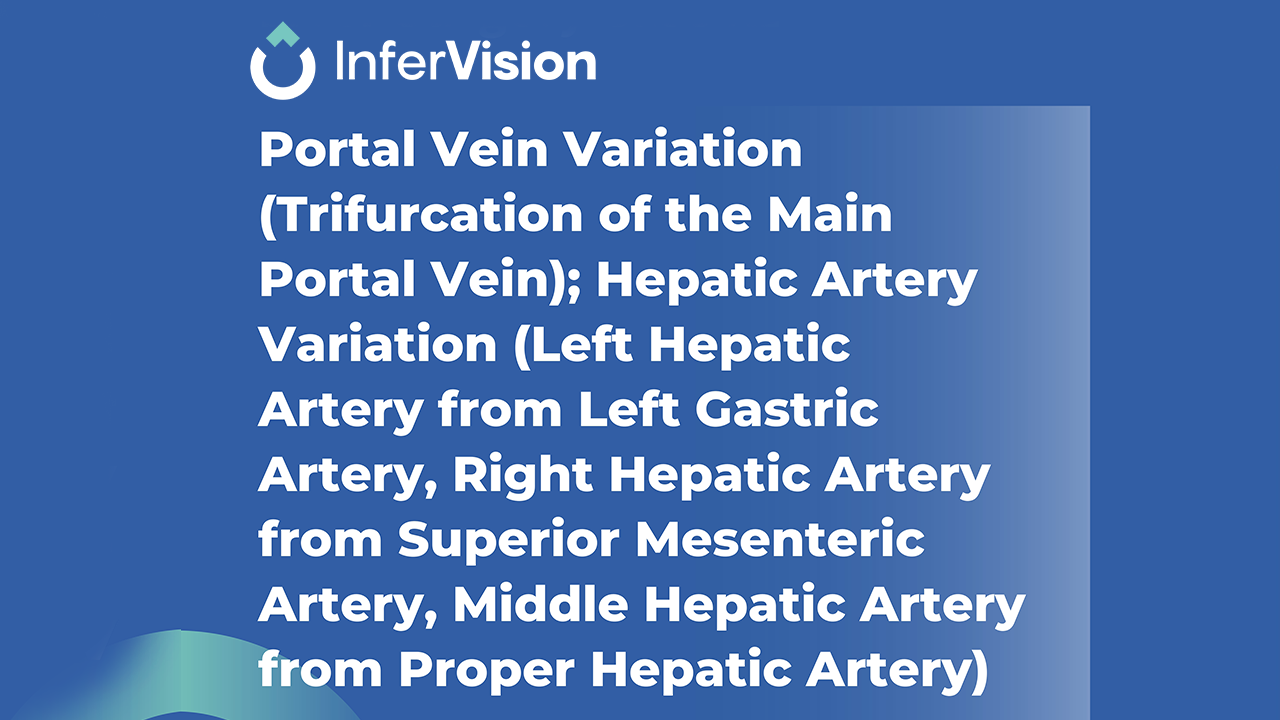
Unlike conventional surgical planning tools that require manual input and lengthy preparation, InferOperate Suite automates the generation of 3D models within minutes. It builds detailed 3D representations of organs —such as lung lobes and segments, liver Couinaud partitions, kidneys—as well as vascular structures, bones, and skin, facilitating both preoperative planning and intraoperative image guidance.
The solution is especially relevant in the treatment of lung, liver, and kidney tumors that pose high surgical risk due to complex anatomy or potential complications. InferOperate Suite supports clinicians by quantitatively analyzing lesions, enabling precise planning of surgical margins and helping reduce complications during surgery and possible recurrence afterward.
Clinical data show that use of InferOperate Suite can shorten preoperative planning time and lower surgical complication rates, yielding cost savings for healthcare systems. In regions with limited medical resources, Infervision’s technology serves as an important tool for expanding access to surgical planning technologies and supporting healthcare systems in strengthening overall care delivery.
“This FDA clearance, alongside our approvals in Europe, the U.K., and China, ensures that surgeons worldwide can rely on AI to improve patient safety, surgical precision, and reduce the burden on healthcare systems,” Infervision stated. “We believe that surgical planning will no longer remain an option available only to a few top tier hospitals but will become an essential tool for surgeons everywhere.”