London, UK - Infervision is proud to announce that its AI-assisted diagnostic solutions for chest, brain, and heart - covering indications for pulmonary nodules, pneumonia, intracranial hemorrhage, coronary artery disease, and chest fractures - have achieved dual certification from the European MDR CE and UKCA. This significant accomplishment strengthens Infervision's position in the European and UK markets and marks a major milestone in its global strategic expansion. Infervision's products also hold certifications from the US FDA, European CE, Japan PMDA, UK UKCA, and China NMPA, securing access to the five major global markets.
Coronary Artery Disease (CAD) is the leading cause of death in Europe, with over 6 million new cases diagnosed annually1. Early and accurate diagnosis is crucial for timely intervention to reduce the risk of heart attacks and strokes. Infervision's AI technology addresses this critical need by enhancing the accuracy and efficiency of diagnoses. Its coronary AI, designed for coronary CT angiography, assists in evaluating coronary artery stenosis, significantly improving diagnostic precision and reducing both reconstruction and reading times, thereby providing substantial clinical value.
Neurovascular diseases, such as strokes and intracranial hemorrhages, are major causes of disability and death in Europe. Annually, over 1.1 million people in the EU are affected by strokes, with more than 12 million living with stroke-related sequelae2. The standard for evaluating stroke thrombolysis time, known as DNT (door to needle time), mandates that the interval from patient admission to thrombolysis should be less than one hour. Infervision, in collaboration with its ecosystem partners and utilising its expertise in 5G, mobile CT, and AI technologies, has developed a 5G mobile stroke unit that enables diagnosis and treatment at the stroke site, already approved and in use in China. Named InferRead CT Rapid Triage, this technology automatically identifies suspected positive cases of intracerebral haemorrhage (ICH), reducing the time clinicians need for diagnose and streamlining workflow triage. This innovation integrates pre-hospital and in-hospital stroke management, enhancing patient outcomes.
Infervision's InferRead CT Lung was incorporated into the UK's National Health framework as early as 2021, offering early screening and detection services for pulmonary nodules. This initiative aids the NHS in reducing the costs associated with lung cancer prevention and treatment. In May 2023, Infervision's AI solutions were once again included in the NHS SBS framework. This ongoing recognition highlights Infervision's acceptance and endorsement by European healthcare systems, validating its service and deployment capabilities in international markets.
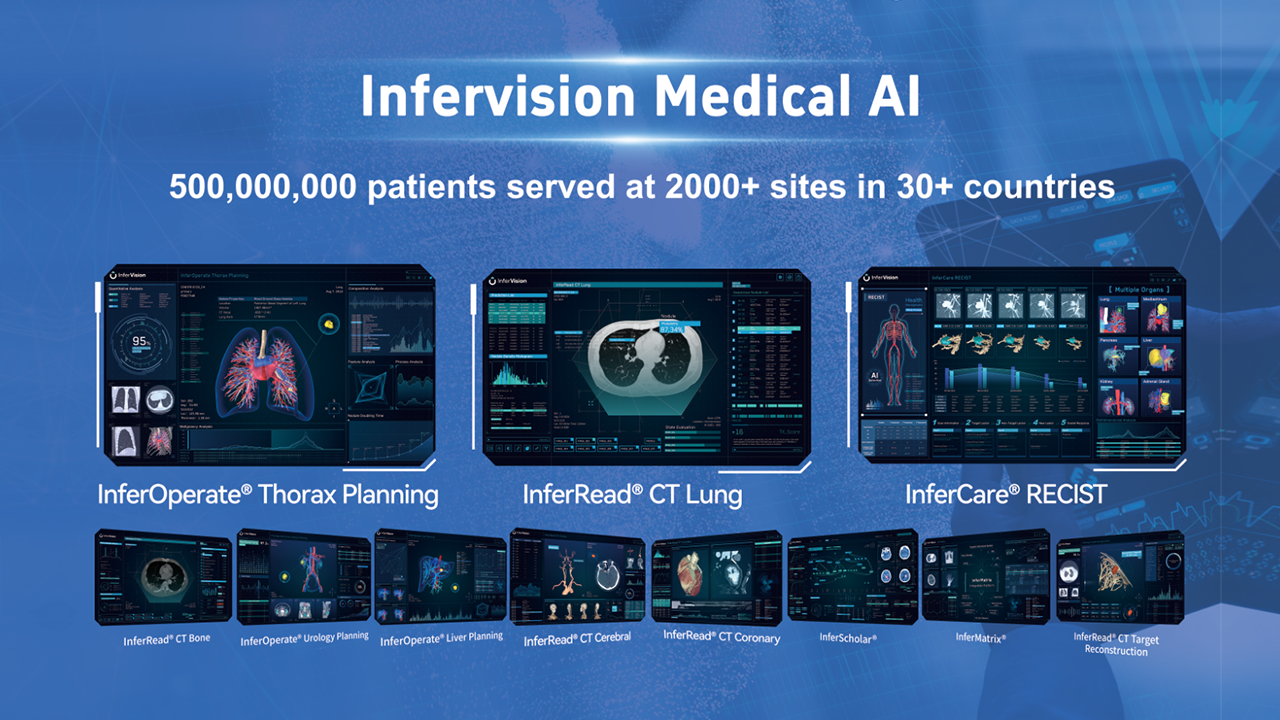
As a global leader in AI medical innovation, Infervision is dedicated to advancing human health through a shared vision. By adopting a vertical and horizontal product strategy, Infervision offers a comprehensive series of AI-assisted software to detect a wide range of anomalies including cancers, infectious diseases, cardiovascular diseases, cerebrovascular diseases and trauma and more. With solutions spanning disease screening and diagnosis (InferRead®), intervention and treatment (InferOperate®), patient health management (InferCare®), and teaching and research initiatives (InferScholar®, InferMartrix, InferEducate), Infervision is the trusted partner for hospitals, other medical institutions, governments, doctors and patients across the globe.
[1] Ehteshami Bejnordi, B., et al. (2023). "Artificial Intelligence in Radiology: Trends and Implications." European Radiology, 33(1), 215-229. DOI: 10.1007/s00330-022-09157-2
[2] European Stroke Organisation (2021). "Stroke Statistics in Europe." ESO Report